5. METHANE CATASTROPHE (Continental Margin Methane Release)
Perhaps the first scientist to have realized
that permafrost and seafloor methane hydrate release may have
played a role in the end-Permian extinction was Doug Erwin (1993),
following the proposal by Paull (1991) tying regression to hydrate
release by depressurization. Erwin indicated that methane and
carbon dioxide (from both the oxidation of exposed continental
shelf organic carbon and methane, "and possibly other sources")
would have contributed to global warming and "possible oceanic
anoxia" (1993, p. 256). Oceanic anoxia is an excellent mechanism
for killing off aerobic marine organisms, but Erwin was clearly
hesitant about invoking it for the end-Permian, hedging his tentativeness
with the words "possible" and "perhaps" (1993,
p. 256).
It may be that Erwin realized that his
model was severely limited by the great length of time over which
he believed the extinction to have taken place. He thought that
the extinction had taken place over a period of about three million
years, "and perhaps as many as 8 million years" (1993,
p. 226). Over such extended lengths of time, neither methane nor
additional carbon dioxide would have had much effect -- if any
-- on atmospheric concentrations of carbon dioxide or ocean chemistry.
What Erwin missed was the importance
-- indeed, the indispensibility -- of the rapid increase of these
gases for the transformation of global climate and marine chemistry.
(The rate of release is crucial. A truck, moving at 100 kilometers,
or sixty miles, per hour, can be extremely dangerous to things
in its path; at a meter -- yard -- per hour, the truck would constitute
little or no danger. Just as the truck's potential threat depends
on its momentum and therefore its speed, so also does the ecological
impact of methane and carbon dioxide depend on their ecological
momentum, or rate of increase. The faster the delivery, the harder
the blow.) Methane released from hydrate slowly (over a period
of many millions of years), for example, would have easily been
consumed by just a small rise in the population of methanotrophs,
which would have increased in response to the greater availability
of their staple, methane.
(Erwin later reduced the amount of time
he thought the extinction had taken, stating that, "There
is, however, little support for claims that the mass extinction
occurred over eight million years, but it is unclear whether the
extinction lasted two million years, 1 million years, or even
less" [Erwin, 1994]. He also came to attribute the extinction
to "synergistic" cause[s]. "Synergistic" is
a ten dollar word [meaning "working together"] with,
in this context, a nickel's worth of content, reflecting a Texas
sharpshooter's lack of specificity. The causes of all mass extinctions
are complex and interactive. However, Erwin further indicated
that "The cause of the end-Permian mass extinction appears
to involve a tangled web rather than a single mechanism."
Erwin identified three "phases"
in this "tangled web," at least as presented in 1994.
The first phase, the onset of regression, involved the drying
out shallow marine basins, reduced habitat for coastal marine
organisms, and increasing climatic variability. During the second
phase, regression accelerated, releasing gas hydrates, and allowing
the erosion and oxidation of marine carbon. In the third phase,
Siberian flood basalts and carbon sources exacerbated climate
instability, producing "an increase in atmospheric carbon
dioxide may have produced oceanic anoxia and global warming."
In this final phase, an earliest Triassic rapid transgression
purportedly destroyed near-shore terrestrial habitats [Erwin,
1994]. Though Erwin invokes the release of gas hydrates in his
tangled web proposal, it is important to note that his central
extinction mechanism is sea level regression, followed by rapid
transgression, rather than methane release.)
At just about the same time that Erwin was developing his regression
scenario, an Australian researcher was coming to similar, but
more focused conclusions about the end-Permian carbon isotope
excursion and its likely consequences. Based on his examination
of numerous ancient ocean basins on the margins of the Australian
continent, Richard Morante discovered a massive shift of carbon
isotopes ranging, in various basins, from -5 to -8. Like Erwin,
Morante traced the carbon isotope excursion to a worldwide regression
caused by the assembly of Pangaea and the slowing of the rate
of the subduction of oceanic plates under that megacontinent (Morante,
1993).
But Morante understood the isotopic excursion
to have been the possible result of "the release of potentially
huge volumes of 13C-depleted methane stored in clathrates in tundra
environments and polar continental shelves," which, together
with increased carbon dioxide released from continental shelf
sediments, would have created a greenhouse world. In addition,
Morante found that the negative carbon isotope excursion took
between 300,000 and 600,000 years, making for a much shorter extinction
event than that contemplated by Erwin. (As noted previously, we
now know that major hydrate methane releases actually take place
in less than 10,000 years.) Basing his estimate of the length
of time needed for post-extinction recovery on the carbon isotope
record, Morante placed the emergence of the planet from its end-Permian
trauma at about the latest Middle Triassic, roughly ten million
years later. Morante also recognized that the end of the Permian
brought a reduction of global oceanic thermohaline circulation,
though he did not specifically tie this to the hydrate methane
release (Morante, 1993).
The continental margin methane proposal
presented here and previously (Vermeij and Dorritie, 1996; Dorritie,
2002) incorporates some of the undisputed facts about the end-Permian
world. It accepts that Siberian Traps volcanism had a powerful
effect on Permian world climate. But rather than the Traps eruptions
being the sole cause of the extinctions, it sees the Traps as
having had an important role, though largely as a trigger for
the release of hydrate methane and the free methane below. Traps
eruptions facilitated this release in two ways: first, directly,
by heating terrestrial permafrost and northern continental margin
sediments. This direct heating would have been by lava flows,
pyroclastic eruptions (superheated, ground-hugging, volcanic dust
clouds), and ash falls, and would have led to the immediate release
of methane from the affected areas. Traps volcanism would also
have directly heated the seawater of the PaleoArctic Ocean (the
northernmost part of Panthalassa; sometimes referred to as the
Boreal Ocean), and that warmed water would have heated seafloor
sediments and released methane from the continental margins across
which it flowed. In addition, by injecting vast quantities of
carbon dioxide into the atmosphere and thereby warming the planet
and the ocean, Traps volcanism would have indirectly heated continental
margin hydrate and released much of its methane.
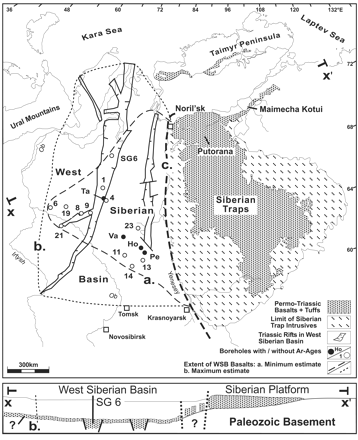
Map of the Siberian Traps. The area to the right of the
dark dashed line ('c') is basically what was known of the extent
of the Siberian Traps until recently, although many geologists
suspected that these massive continental flood basalts were even
more extensive. This part of the Traps consisted of basalt lave
flows and tuffs (airborne ash), plus "intrusives": magma
which never reached the surface, but solidified underground. Recent
drilling in the area (outlined by dashed line 'a') to the left
of the dark dashed line (part of the West Siberian Basin), however,
has revealed that Traps volcanism extended much further west,
but was buried under younger sediments. The total extent of the
Traps may even extend as far as the dotted line, 'b.' The elongated
features enclosed by solid lines in the West Siberian Basin are
grabens (literally, graves), which are great rifts where blocks
of crust have dropped down relative to the crust around them.
The lower diagram (b.) provides a cross-section which shows this
more clearly. (Lake Tahoe, on the California-Nevada border, lies
in a graben, with mountains surrounding the down-dropped block
of crust.) The cross-section shows that magma either erupted in,
or flowed down into the basin, the possibility of which was previously
proposed by Vermeij and Dorritie, 1996. This would have rapidly
released methane from permafrost and continental margin hydrates.
(Reichow, 2002)
Traps volcanism would have had important
additional consequences unrelated to continental margin methane
release, but providing in themselves mechanisms for altering the
course of life on Earth. Volcanic gases would have been oxidized
and hydrated, producing huge quantities of acid, the most important
of which would have been carbonic and sulfuric acids. These would
have adversely impacted both terrestrial and marine organisms.
The outpouring of volcanic ash would
have had additional major effects, largely in the ocean. There
the ash would have provided fertilizer for phytoplankton. While
underfertilization is bad -- it leads to starvation of the underfertilized
organisms -- overfertilization and exceptional phytoplankton productivity
may be bad as well. As phytoplankton populations explode due to
overfertilization, other organisms are deprived of the oxygen
used by the decomposers of dead phytoplankton. Volcanic ash, therefore,
by providing fertilizer, would have facilitated oceanic dysoxia
in the deeper ocean.
Finally, of course, the great amounts
of water vapor and carbon dioxide emitted by the volcanism would
have warmed the planet. Any northern sea ice that had not been
melted directly by the heat of the volcanism itself, by seawater
warmed by that volcanism, or by the darkening of polar ice by
volcanic ashfalls, would have melted as a result of the increased
polar temperatures associated with global warming. The melting
of sea ice would have produced fresher and warmer water at and
near the pole, causing a significant disruption of global thermohaline
circulation.
But the major effect of Traps volcanism,
in this scenario, would have been to rapidly release continental
margin methane. The release of margin methane would have had several
important effects, each of which would have had serious consequences
for living things.
Methane itself
First, methane itself is, like carbon dioxide, an asphyxiating
gas, depriving aerobic organisms of needed oxygen. When released
in the ocean, it would have impaired the metabolism of aerobic
marine organisms, and, in sufficient concentrations, would have
caused death. Although, upon reaching the atmosphere, methane
could have had similar effects on non-marine organisms, its concentrations
would have been unlikely to do much harm, because methane is lighter
than air and would have been easily dispersed by winds.
Methane oxidation and oceanic anoxia
As methane gas from continental margins moved upwards through
the overlying sediment, some would have been oxidized by the (anaerobic)
methanotrophic organisms which lived in the top twenty meters
or so below the sediment-water interface. The actual oxidation
(uptake of electrons) of this methane would have been accomplished
though the use of sulfate (SO¸4^2), not oxygen itself,
because all of the dissolved oxygen would have been consumed in
the top few centimeters of sediment. The oxidation of the methane
would have resulted in the removal of oxygen from the sulfate,
and the production of sulfide (compounds with sulfur but no oxygen),
most notably as hydrogen sulfide (H¸2S) gas and pyrite (iron
sulfide, FeS¸2).
Once the remaining methane escaped from
the sediment into the ocean itself, more would have been consumed
by aerobic organisms. One gaseous end product of these reactions
would have been carbon dioxide. Thus the release and oxidation
in the water column of continental margin methane would have both
drawn down the oxygen and increased the carbon dioxide (causing
death by carbon dioxide poisoning -- hypercapnia -- in susceptible
organisms: Knoll, 1996).
The carbon dioxide also would have combined
with water to form bicarbonate ions (HCO^3), which reacted
chemically with dissolved calcium to produce calcium carbonate.
This presumably was the main source of the "anomalous carbonates"
observed by Grotzinger and Knoll (1995). Carbonates of similar
origin, "in the form of chimneys and vent linings,"
can be found today along the top of Hydrate Ridge offshore from
the Pacific Northwest, where methane escapes through faults from
the seafloor. Photographs at the site reveal "muddy chunks...of
alternating layers of pure-white methane hydrate, sediments, and
limestone. But despite the limestone casing and the activities
of the vent organisms, surprising quantities of methane escape
into the surrounding ocean water" (Suess, 1999).
Likewise, at the end of the Permian,
large quantities of methane would have escaped oxidation and bubbled
upwards through the seawater. When this methane reached the atmosphere,
oxidation would have continued, slightly lowering levels of oxygen
but significantly raising levels of carbon dioxide. Obviously,
the amount of oxygen drawn down, and the amount of carbon dioxide
produced, depended on the quantity of methane released.
In the oceans, the drawdown of oxygen
would have led to dysoxia, or, if the size of the methane release
was great enough, to local or widespread anoxia. This would have
had harmful effects on aerobic organisms, which require oxygen
for survival. The effects would have ranged from mild physiological
consequences to death, depending on the extent of the dysoxia
or anoxia. Where anoxia became pervasive and enduring, as in the
deep ocean, it would have been a major cause of the end-Permian
marine extinction.
With anoxic conditions in the deeper
ocean, anaerobic organisms, including methanogens and sulfate-reducers,
would have thrived. Their presence in an anoxic ocean would have
given rise to two gases. One, obviously, was methane, and the
methanogen bloom (population explosion) which would have taken
place would have provided a continuous supply of methane both
to the ocean itself (where much would have been consumed by methanotrophs)
and to the atmosphere. The increased and sustained production
of methane would have protracted marine anoxia and sustained high
methane levels in the atmosphere.
Hydrogen Sulfide
Meanwhile the sulfate-reducers would have pumped out a second
gas, which for them is a waste gas: hydrogen sulfide (H¸2S),
toxic both to marine organisms and those along affected coasts.
The toxicity of hydrogen sulfide, in fact, is comparable to that
of hydrogen cyanide (HCN), which is used in gas chambers. This
toxicity is due to hydrogen sulfide's ability to combine with
the iron found in numerous organic molecules, such as hemoglobin.
Even the sulfate-reducers themselves are vulnerable to hydrogen
sulfide poisoning, though the hydrogen sulfide gas typically combines
rapidly with iron ions found in seawater, producing an insoluble
black (iron sulfide) residue which colors the nearby sediments
(Brock and Madigan, 1988, p. 578).
In addition, hydrogen sulfide reacts
with water to produce sulfuric acid, which would have pushed the
oceanic acidity-alkalinity balance toward lower pH (more acidic)
values, inflicting harm on acid sensitive organisms. The quantities
of hydrogen sulfide that would have been produced in an anoxic
ocean are not negligible: for each sulfate ion that is employed
in the sulfate-reduction process, a molecule of hydrogen sulfide
is produced (see Box).
|
Sulfate Reduction
Sulfate reduction produces hydrogen sulfide (and several other
ionic compounds, including the bicarbonate ion, HCO^3) according
the following generalized equation. The equation employs a sort
of "averaged" organic compound as a starting point.
This compound employs the "Redfield ratio" of carbon
to nitrogen to phosphorus -- 106 C: 16 N: 1 P -- that is typical
of many organic compounds.
C¸106H¸263O¸110N¸16P¸1
+ 53 SO¸4^2 +
14 H¸2O Æ
("averaged"
organic compound) + (sulfate) +
(water) (yields)
53 H¸2S +
106 HCO^3 + HPO¸4^2 +
16 NH^4+ + 14
OH^
(hydrogen sulfide) + (bicarbonate) + (phosphate) +
(ammonium) + (hydroxyl)
(Kempe and Kazmierczak,
1996, Figure 4, p. 74, as modified from Kempe, 1990; the "phosphate"
is monohydrogen orthophosphate.)
Some of the H¸2S
is oxidized during upwelling, but some eventually is combined
with dissolved iron ions to form pyrite (FeS¸2), which
is insoluble and precipitates out. In addition, the excess alkalinity
(represented in the equation by the bicarbonate ion, HCO^3)
combines with dissolved calcium ions to form calcium carbonate,
which also precipitates out. This may be the source of some of
the Permian-Triassic boundary "anomalous carbonates"
noted by Grotzinger and Knoll (1995), and invoked by Knoll and
co-workers (1996) in their oceanic overturn scenario.
|
Even today the toxicity of hydrogen sulfide
is environmentally evident. It is responsible for the death of
many nearshore fish and invertebrates along more than 200 kilometers
(120 miles) of the Namibian coast of southwest Africa (Weeks,
2002; Weeks, 2004). In December of 2003, more than two hundred
Chinese perished and some ten thousand needed medical assistance
from a release of hydrogen sulfide triggered by an explosion at
a natural gas field. An area some 25 square kilometers (10 square
miles) in extent was affected.
The Chinese deaths point up one important
characteristic of hydrogen sulfide: it is heavier than air (though
not very much so). This allows it to hug the ground and kill organisms
which live on or below the surface. Flying organisms such as birds
and larger insects are therefore less vulnerable to hydrogen sulfide
releases. (Along the Namibian Coast, rock lobsters flee from the
sea bed to the shore during hydrogen sulfide emission events,
while seabirds scavenge the floating corpses of gas victims; Weeks,
2000; Weeks, 2004.) Of course, the gas does dissipate fairly rapidly,
especially with sufficiently strong air movement, but it may do
so after having left much death in its wake.
Special conditions give rise to the hydrogen
sulfide emissions along the Namibian Coast. Cold, highly oxygenated
water from the periphery of Antarctica moves north and wells up
along this coast. Known as the Benguela Current, the water carries
nutrients up from the deep ocean. This results in very high marine
productivity, and consequently (though perhaps counterintuitively)
in the drawing down of oxygen in the water column at fairly shallow
depths as aerobic organisms decompose the descending organic debris.
Continental shelf dysoxia and even anoxia ensue. In the diatomaceous
ooze (the accumulation of uncountable numbers of diatom skeletons)
which constitutes the ocean floor, the sulfate-reducers employ
sulfate from ocean water to consume additional organic debris,
discarding hydrogen sulfide as a waste product (Weeks, 2002; Weeks,
2004).
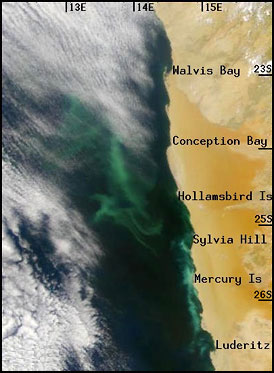 Hydrogen sulfide off the Namibian
coast. The green color indicates
concentrations of hydrogen sulfide. The coast (a desert) is to
the right, the ocean to the left, with clouds above. (Weeks, 2002)
Hydrogen sulfide off the Namibian
coast. The green color indicates
concentrations of hydrogen sulfide. The coast (a desert) is to
the right, the ocean to the left, with clouds above. (Weeks, 2002)
If marine productivity were not so high,
oxygen would not be so depleted, and much of the hydrogen sulfide
would be oxidized in the water column. But along the Namibian
Coast, there is not sufficient oxygen to do so despite the highly
oxygenated current that sweeps by, and much hydrogen sulfide escapes
into the atmosphere, bringing the harsh and toxic smell of rotten
eggs -- and sulfuric acid aerosols -- to coastal villages (Weeks,
2002). Interestingly, some of the hydrogen sulfide is itself oxidized
in the water column and thereby helps draw down any remaining
dissolved oxygen. Furthermore, hydrogen sulfide is the one gas
of those discussed herein (including carbon dioxide and methane)
whose dramatic toxic impact on living things can be easily observed,
although this impact occurs in a remote part of the world.
|
Microbes and Phosphate
One quite curious organism
found 100 meters (yards) below sea level in the seafloor muds
of the Namibian coast looks like a short string of tiny white
pearls, each slightly larger than the period at the end of this
sentence. When these "pearls" were first observed in
shallow sediment samples, and then identified as bacteria, they
produced a reaction of incredulity among many scientists because
they were vastly larger than any bacterium previously known.
They were, in fact, about 100 times larger across than the average
bacterium, and about three million times its volume. The identification
proved accurate, however: the organisms actually enclosed huge
nutrient storage spaces (vacuoles). Inside the vacuoles were,
among other things, tiny globules of elemental sulfur (hence
the whitish color), obtained from the surrounding sulfidic waters.
Much of the "pearl" therefore was sulfur; only about
2% was the enclosing organism (Schulz, 1999).
This bacterium, named
Thiomargarita namibiensis ("sulfur pearl of Namibia"),
accumulates in anoxic conditions large quantities of sulfur which
it oxidizes for energy by the use of nitrate. The sulfur apparently
is stored to get the organism through long periods when sulfur
is in short supply. Similarly, T. namibiensis also stores nitrate,
which is only available during periods when major storms bring
oxic waters, which carry nitrate, down to the seafloor (Schulz,
1999).
Further investigation
of this unusual organism revealed that it possessed an "auxiliary
mechanism" for getting through hard times. In addition to
storing sulfur and nitrate, it also stores polyphosphates, complex
molecules containing phosphate (PO¸4^3). During times
when storms carry nitrate to the depths, carbon is oxidized and
phosphate is taken up and stored as polyphosphate. When quieter
-- and anoxic -- conditions return, T. namibiensis apparently
breaks down the polyphosphate for energy, allowing it to take
up and accumulate acetate (likely stored as glycogen, a major
energy source for many organisms), while dumping phosphate in
the process (Schulz and Schulz, 2005).
These mechanisms help
the organisms cope with the highly variable (and highly seasonal)
upwelling conditions associated with the Benguela Current, and
the turbulence created by intermittent major storms off the Namibian
coast. Similar mechanisms are employed by organisms used in wastewater
treatment plants to remove phosphates (Schulz and Schulz, 2005).
The phosphate dumped
by T. namibiensis and similar large sulfur bacteria (Thioploca
and Beggiatoa, all members of the gamma proteobacteria phylum)
seems to be the source of contemporary seafloor phosphate formation,
often found upwelling areas. Presumably these organisms or their
ecological equivalents (organisms which make their livings in
a similar fashion) were responsible for the world's major phosphorite
deposits (Schulz and Schulz, 2005), including the Phosphoria
Formation of the US Mountain states (Idaho, Wyoming, Utah, Montana,
and small parts of Colorado and Nevada). (The phosphate may have
been blown from its place of origin to its current location,
perhaps after the drying of the shallow sea in which it formed.)
The Phosphoria Formation is of Late Permian age.
Because phosphate dumping
by large sulfur bacteria occurs only during episodes of anoxia,
the existence of this formation indicates that it originated
when deeper water anoxic conditions prevailed in a shallow sea
in what was then the western coast of Pangea. If those organisms
which were engaged in similar activities in the Permian were
like those of today, they also would have required periodic infusions
of oxygen or nitrate, and thus Late Permian surface waters may
well have been oxic, at least in this part of the world. (As
of early 2007, fossils which may be those of an ancient relative
of Thiomargarita have been found in 600 million-year-old phosphorite
[Bailey, 2007; Donoghue, 2007]. If this identification is confirmed,
it means that Thiomargarita-like organisms would certainly have
been present in Late Permian waters.)
|
Hydrogen sulfide may have played a major role in the end-Permian
extinction. As oxygen levels in the Permian world ocean declined,
the sulfate-reducing organisms would have gradually expanded outward
from those limited anoxic habitats (in seafloor sediments and
anoxic basins) to which they had been confined by the presence
of oxygen elsewhere. As sulfate-reducers came to dominate the
latest Permian and Early Triassic ocean, the hydrogen sulfide
they produced would have contributed to the extinction of aerobic
marine organisms.
The record of such an expansion of sulfate-reducing organisms
has, in fact, probably already been identified: it is the huge
changes in sulfur isotopes that occurred at the end of the Permian,
as noted by Kaiho (2001). Although Kaiho and his co-authors thought
that the sulfur isotope excursions were due to an enormous impact,
it seems more likely that the excursions they observed were the
consequence of the greatly increased activities of sulfate-reducers.
As with carbon, living things prefer to use the lighter isotope
of sulfur. In preferentially using the oceanic sulfate with the
lighter rather than the heavier isotope (that is, ^32S
rather than ^34S), the lighter isotope would have become more
prominent in the sulfide waste product of the sulfur-reducers
and the heavier isotope more prominent in the remaining sulfate.
In addition, however, the hydrogen sulfide
may have breached its marine confinement and escaped into the
atmosphere (Kump, 2005). Numerous detrimental consequences would
have followed, quite apart from the direct poisoning of terrestrial
organisms. In low concentrations, hydrogen sulfide has the interesting
ability to cause mice to enter a hibernation-like or suspended
animation-like state (Blackstone, 2005). In such a condition,
they would become easy victims for predators, assuming that the
predators themselves did not drop into the same lethargic state
as their prey. (The effect of low concentrations of hydrogen sulfide
has only been tested on mice, but similarly low concentrations
may have similar effects on other mammals, or other animals.)
But there were no mice around in the latest Permian/Early Triassic:
rodents evolved almost two hundred million years later. Nonetheless,
the mammal-like reptiles (therapsids) that did exist may have
been likewise affected, and perhaps other organisms as well.
More important than its sleep-inducing
qualities would have been two other effects of atmospheric hydrogen
sulfide. Hydrogen sulfide readily combines with the hydroxyl (OH^)
ions in the atmosphere. Hydroxyl is the major methane-limiting
component of the atmosphere; without it, methane concentrations
can increase almost without limit. More importantly, the depletion
of hydroxyl increases the longevity of methane in the atmosphere,
so that instead of having a residence time (lifetime) in the atmosphere
of less than ten years, methane can stick around much longer,
significantly extending its powerful greenhouse gas warming. If
hydrogen sulfide did escape confinement at the end of the Permian,
global temperatures would have skyrocketed (Kump, 2005).
Furthermore, atmospheric hydrogen sulfide
also combines with and thereby consumes ozone (O¸3). Today
there are two places in the atmosphere where important quantities
of ozone may be found. One is near ground level, where automobiles
and trucks produce often hazardous concentrations of ozone-laden
smog. There were no internal combustion engines 250 million years
ago, so there were no important concentrations of ozone at ground
level at that time.
But ever since the earliest phytoplankton
started pumping significant quantities of oxygen into the atmosphere,
perhaps some 2.5 billion years ago, high altitude ozone (the "ozone
layer") has been extremely important to life on Earth. This
ozone absorbs and therefore blocks ultraviolet (UV) light from
the sun, which is quite dangerous for living things. Ultraviolet
light is high energy radiation; as such, it can tear apart organic
molecules and cause mutations and death to organisms.
In fact, it is generally presumed that
the lack of ozone in Earth's early atmosphere inhibited the evolution
of complex organisms and prevented the expansion of living things
onto the land, where they would have been exposed to destructively
high levels of ultraviolet light. Only with the rise of atmospheric
ozone could organisms have left the protection of their aquatic
environments (water also protects against ultraviolet light) and
venture out onto the land. With sufficient hydrogen sulfide being
pumped into the atmosphere at the end of the Permian and in the
beginning of the Triassic, the protection of the ozone layer would
have been removed, and terrestrial and shallow-water-living aquatic
organisms would have suffered mutation and death (Kump, 2005).
Although a hydrogen sulfide release would have had these several
major environmental consequences, however, the release scenario
does have a number of serious difficulties. First, the scenario
as presented relies on atmsopheric release through regions of
intense oceanic upwelling. Such regions, where strong undersea
currents carry nutrients from the deep ocean to shallow depths,
are comparatively uncommon. Upwelling regions constitute only
about 0.1% of the surface area of today's ocean (Kump, 2005).
(The Benguela Current, upwelling along the coast of Namibia, is
an example.) Nonetheless, they are critical to the scenario because
they produce the eutrophic (highly fertilized) conditions that
result in near-surface anoxia.
But the anoxia and euxinia of the Permian
ocean presumably had its origin in its stratification (ultimately
due to global warming). A stratified ocean would likely have had
limited upwelling, perhaps not enough to have allowed significant
amounts of hydrogen sulfide to reach the ocean surface and thereby
the atmosphere. Alternatively, hydrogen sulfide releases could
have been confined to specific coastal areas near intense upwelling
regions, just as with today's Namibian coast. In addition, anoxic
conditions can permit the depletion of an essential nutrient,
nitrate (Falkowski, 2004; Kuypers, 2005). So even if the amount
of Permian ocean upwelling was equivalent to that of today (as
the Kump, 2005, proposal assumes), that upwelling may have lacked
the needed nitrate to induce eutrophic surface water conditions,
anoxia, and hydrogen sulfide release.
Finally, there is the matter of the level of Late Permian oxygen.
Hydrogen sulfide escape from ocean confinement depends in part
on the amount of oxygen in surface waters, which largely depends
on the amount of oxygen in the atmosphere as it exchanges gas
with the ocean across their interface. (The amount of surface
water oxygen which is directly contributed by its phytoplankton
inhabitants is relatively small.) Oxygen in the surface waters
limits both the expansion of sulfate-reducers, which are anaerobes,
and hydrogen sulfide itself, which it oxidizes.
If the atmospheric level of oxygen in
the Late Permian were low, therefore, it would have been easier
for hydrogen sulfide to breach containment in the ocean. But oxygen
levels were quite high, probably as high as they have ever been
(at 30 to 35%: Berner, 2001), well into the Permian. The question
is how long those high oxygen levels persisted. At the end of
the Permian, oxygen levels were possibly much lower, dropping
perhaps as low as 16% (Berner, 2002; Huey and Ward, 2005), though
a more recent study suggests a range of between 33/34% and 15%,
with a most probable value being about 20% (Berner, 2007). But
when did the transition take place, and how rapidly did it occur?
Unfortunately, the number of reliable data points across the tens
of millions of years of the Permian is limited, and does not present
a clear picture. But there may be another way of addressing the
issue.
Atmospheric oxygen levels depend on several
factors. Among them are the activities of terrestrial green plants,
the activities of phytoplankton, and the rate at which carbon
(which would combine with oxygen to form carbon dioxide) is removed
from contact with oxygen by burial in ocean sediments (called
carbon export). There is no evidence of any significant or unusual
changes in terrestrial flora in the Permian prior to the end-Permian
extinction. Nor is there any indication of changes in the rate
of carbon burial until we encounter the brown and black shales
of Permian-Triassic boundary rocks. Data on Permian phytoplankton
is minimal.
In sum, there is no significant evidence
of biological or ecological changes in the Permian that would
have affected the level of atmospheric oxygen, with the possible
exception of an extinction event about five million years before
the end of the Permian (the end-Guadalupian extinction: Stanley
and Yang, 1994; Racki, 2003). Lacking any compelling evidence
for changes in those factors which would have affected atmospheric
oxygen levels, therefore, the most reasonable assumption is that
atmospheric oxygen levels nearing the end of the Permian were
pretty much the same as at its start, and that it was the Permian
extinction event itself which was responsible for the plummeting
of those levels. If this reasoning is correct, then hydrogen sulfide
releases to the atmosphere would have been one of the many effects
of the end-Permian catastrophe (although a quite deadly one),
rather than a cause of the catastrophe per se.
Despite these difficulties, the hydrogen
sulfide release scenario prompts some further comments. First,
once the end-Permian ocean had become largely anoxic (and in anoxic
areas, devoid of aerobic organisms), it would have been quickly
resettled by anaerobes such as the methanogens and sulfate-reducers
(which produce hydrogen sulfide). Sulfate-reducers, not being
quite as strict anaerobes as methanogens, would have expanded
into areas where there were low concentrations of oxygen (highly
dysoxic areas), from which methanogens would have been excluded.
But even in fully anoxic areas, sulfate-reducers would have outcompeted
methanogens for certain important nutrients (specifically for
hydrogen molecules and acetate: Brock and Madigan, 1988), though
some methanogens can use other, less plentiful nutrients. Consequently,
the sulfate-reducers would have become the main inhabitants of
the anoxic deep ocean, sharing it with more limited numbers of
methanogens. No wonder sulfidic (euxinic) conditions developed
in the Early Triassic ocean (see the discussion of the Black Sea,
below, in the Early Triassic Aftermath section).
Second, because hydrogen sulfide readily combines with iron, it
would have effectively scavenged iron from much of the ocean,
combining with it and sending it to the bottom as iron pyrite
(FeS¸2). Iron is an essential nutrient, and one which
already exists in only limited quantities in the ocean. (That
is why "iron fertilization" is being examined as a strategy
for dealing with the atmosphere's excess carbon dioxide. Provided
to phytoplankton, it creates "blooms" which, because
the phytoplankton use carbon dioxide, helps reduce its presence
in the air.) With iron availability already limited, marine organisms
would have faced conditions of iron starvation once hydrogen sulfide
became plentiful.
Third, it seems quite unlikely that no
hydrogen sulfide would have escaped oceanic containment, because
it escapes in places today despite our very well oxygenated ocean.
Once in the atmosphere in significant quantities, hydrogen sulfide
would have interacted with hydroxyl ions (OH^), and helped
block the destruction of methane by those ions (that is, it would
have increased the methane residence time), allowing faster, greater
atmospheric warming. As the atmosphere's warmth penetrated the
ocean, stratification would have increased (or been maintained),
and the sulfate-reducers would have thrived. Thus, there could
have been a kind of methane-hydrogen sulfide partnership, with
each gas enhancing the presence of the other, until other factors
(such as the drawdown of atmospheric carbon dioxide via increased
weathering of silicate rocks) brought the era of the relationship
to a close. Such a partnership may have also existed at other
times of ocean anoxia, as indicated by significant negative sulfur
isotope excursions: in the Early Cretaceous (120 to 100 million
years ago: Paytan, 2004), and during the Paleocene-Eocene Thermal
Maximum (55 million years ago: Paytan, 1998; Faul, 2005).
Seven important gases:
summary table
(Please remember
that ¸ indicates a subscript; ^ indicates a superscript.)
| Gas |
Symbol |
Significance |
| Oxygen |
O¸2 |
A waste
product of oxygenic photosynthesis. Chemically active; it combines
readily with other elements. Not present in Earth's early atmosphere
because oxygenic photosynthesizers (specifically, phytoplankton)
had not yet evolved. Today's atmospheric level (about 21%) is
maintained by the continuing activity of phytoplankton and terrestrial
green plants. Required by all aerobic organisms. Highest level,
during the Permian, was 30-35%. This level fell rapidly at the
end of the Permian and during the Early Triassic, to perhaps
12%. |
| Water vapor |
H¸2O |
The primary
greenhouse gas, but not one that humans have much to do with,
because of the vast amount of water covering the surface of the
Earth. This surface water is freely exchanged with the atmosphere
(in geologists' terms, atmospheric water vapor and surface water
are "in equilibrium"). The quantity of water vapor
in the atmosphere is determined by temperature, and varies greatly
from place to place. When temperature rises, surface water evaporation
increases, raising the amount of water vapor in the local atmosphere;
when temperature drops, water vapor condenses out, as rain or
snow. Global warming allows the total quantity of atmospheric
water vapor to increase. |
| Carbon
dioxide |
CO¸2 |
The main
greenhouse gas largely under the control of human beings. It
accounts for most (roughly two-thirds) of global warming. Though
there is a natural level of atmospheric carbon dioxide (with
some variability over time), human beings are increasing the
presence of this gas in the atmosphere at an unprecedented and
likely catastrophic rate. This anthropogenic (human-caused) carbon
dioxide will decline with the inevitable depletion of fossil
fuels, but global warming will nonetheless increase for some
time thereafter. Once the dumping of carbon dioxide into the
atmosphere ceases, the amount of carbon dioxide will decline
over the centuries, but an appreciable quantity (perhaps 7%)
will still be around in 100,000 years. |
| Methane |
CH¸4 |
The primary
constituent of "natural gas," this greenhouse gas is
often associated with petroleum or coal, and can cause asphyxiation
or explosions in coal mines. Found in huge quantities in icy
lattices (hydrates) in seafloor sediments, it can be released
by depressurization or warming. Has considerably greater global
warming potential than carbon dioxide. Roughly one-third of current
global warming is attributable to atmospheric methane (though
little of the present methane comes from hydrate). |
| Hydrogen
sulfide |
H¸2S |
A highly
toxic gas, known by its rotten egg smell. Produced by sulfate-reducing
organisms in anoxic marine conditions, it readily combines with
iron in the blood of aerobic organisms, and, in sufficient quantities,
kills them. |
| Hydroxyl |
OH^ |
This ion
(electrically charged molecule) readily combines with both methane
and hydrogen sulfide (among other atmospheric gases). Its presence
in the upper atmosphere prevents the accumulation of much methane. |
| Ozone |
O¸3 |
With the
rise of atmospheric oxygen beginning about 2 1/2 billion years
ago, this gas -- composed of molecules with three oxygen atoms
-- was also produced. Ozone is found both in the lowest layer
of the atmosphere, the troposphere, and the next highest layer,
the stratosphere (about 10 to 50 kilometers, or 6 to 30 miles
high). Much of the ozone in the troposphere today is produced
by internal combustion engines and electrical devices, and it
contributes significantly to smog. In the stratosphere, however,
though it is only a very minor constituent, ozone absorbs much
solar ultraviolet radiation, thus protecting living things from
this highly energetic, extremely harmful radiation. (Water also
protects against ultraviolet radiation, which is why life arose
in the oceans. But only the rise of atmospheric ozone allowed
living things to colonize the land.) |
(Having completed this table,
I was struck by how many of these gases are often the waste products
of the activities of various organisms: not only is oxygen the
waste product of oxygenic photosynthesizers, but also, carbon
dioxide is the waste product of animals and aerobic decomposers;
methane, the waste product of methanogens; and hydrogen sulfide,
the waste product of sulfate-reducers!)
Ozone
The ozone is the great
protector of non-marine life. Earth's early atmosphere contained
no free oxygen (O¸2), and therefore no ozone (O¸3).
Back then, any free oxygen that was somehow spontaneously produced
would have quickly combined with other chemicals and would have
disappeared from the atmosphere. Only with the origin of life
and the subsequent evolution of phytoplankton in the oceans was
enough free oxygen continuously produced that it was able to accumulate
in the atmosphere. Thus, today's level of atmospheric oxygen (21%
of the air) is entirely due to the activity of phytoplankton and
terrestrial green plants.
Along with the rise of
free oxygen in the atmosphere came ozone. Stratospheric ozone
absorbs much solar ultraviolet light, deadly radiation which causes
genetic mutation and death to exposed living things. But once
there was sufficient ozone in the ancient stratosphere, living
things which previously existed only in the world's oceans, where
they were protected by water, could begin to move onto the surface
of the land. Though some single-celled organisms managed to make
the evolutionary transition to land more than a billion years
ago, terrestrial green plants took many hundreds of millions of
years longer, as the level of atmospheric oxygen -- and ozone
-- increased.
By the Permian, atmospheric
oxygen levels were at an all time high, and the creatures of the
land were relatively well (but not completely) protected from
ultraviolet radiation. Then came the end-Permian volcanism, spewing
out halogen gases (fluorine, chlorine, and bromine), helping deplete
stratospheric ozone. Methane would have made its own contribution
to ozone depletion. But significant destruction of the ozone layer
would likely have come about as an indirect consequence of a major
hydrogen sulfide release.
Both methane and hydrogen
sulfide destroy ozone, but because hydrogen sulfide is a much
heavier gas, it tends to destroy the ozone closest to Earth's
surface. Being much lighter, however, methane can rise into the
stratosphere. On its way, however, methane reacts with other gases
and can be depleted or destroyed. But hydrogen sulfide is also
a chemically active gas, and it can combine with and neutralize
those gases which would interfere with methane's rise into the
stratosphere. Despite its being largely confined to the lowest
level of the atmosphere, therefore, hydrogen sulfide can facilitate
the destruction of stratospheric ozone by methane. In fact, this
scenario has been proposed as a significant killing mechanism
in the end-Permian extinction (Lamarque, 2007), and the discovery
of spores apparently damaged by ultraviolet radiation seems to
confirm it (Visscher, 2004).
But back to methane itself. The quantities
of methane that would have been released at the end of the Permian
would have combined with and slightly drawn down atmospheric oxygen.
More importantly, a large methane release would have inhibited
the production of oxygen both from marine phytoplankton, via altered
oceanic chemical and biological conditions, and from terrestrial
plants, by the increase of acid rain. Reduced oxygen production
would have gradually lowered the atmospheric oxygen level, causing
the metabolic impairment of terrestrial aerobic organisms.
Compounding the effects of low oxygen
would have been the increase in carbon dioxide, producing further
impairment or death by hypercapnia to sensitive organisms in the
surface ocean (which exchanges gas with the atmosphere), and physiological
damage or death to organisms along affected coastlines. Carbon
dioxide is heavier than air, and therefore does not dissipate
as readily as methane, which is lighter than air, does. Its great
killing power was demonstrated by the deaths of villagers and
cattle unfortunate enough to be in its path when it erupted out
of the bottom waters of Lake Nyos in Cameroon, Africa in 1986.
An estimated 1800 people and 6000 cattle were asphyxiated in the
eruption. Lake Nyos fills a volcanic crater; the high concentrations
of carbon dioxide in its bottom waters result from the continual
seepage of that gas from the magma chamber below.
High marine levels of carbon dioxide
have a further consequence. They produce the relatively mild carbonic
acid, which nonetheless has the ability to erode calcium carbonate
in shells, and to inhibit the production of calcium carbonate
in those organisms that employ it in the creation of their shells.
Global Warming
But the most important consequence of a colossal methane release
would have been the warming of the planet by the greenhouse gas
methane and its successor, carbon dioxide. We tend to underestimate
the impact of global warming on other organisms. This probably
stems, at least in part, from our own adaptability to the variable
temperature of changing seasons, though we are assisted in our
adaptation by adding or removing clothing and the artificially
warmer or cooler environments provided by fire; gas, electric
or oil heating; and air conditioning. In the more affluent parts
of the world, mortality from exceptional hot or cold spells tends
to be minimal, and generally affects only socially marginalized
people: the old, the homeless, the poor.
Other organisms are not so fortunate
as affluent human beings. Though some organisms can grow heavier
coats for winter and shed them in spring, or employ other survival
strategies during cold, heat, or drought (seed production, leaf
loss, hibernation, estivation -- that is, reduced activity during
hot spells -- and so on), many have little or no ability to survive
unusual warmth or cold. Nonetheless, the impact of global warming
has been popularly assumed to be something which will only gradually
encroach upon other organisms, and cause extinction only in unusual
circumstances. Instead, habitat destruction has generally been
presumed to be the major human activity which will adversely affect
our fellow species.
We no longer have any excuse for such
naiveté. A careful examination of a large number of species
in numerous parts of the planet projects that a stunning portion
of them will be "committed to extinction" in just 50
years, with only modest global warming (Thomas, 2004). "Committed
to extinction" means that, in the language of poet Pedro
Pietri (1968?), "their names [are] listed in the telephone
directory of destruction," that is, the book of death. It
does not mean that 50 years from now all these "committed"
species will be gone, but rather that they will no longer have
a habitat in which they can survive. The demise of the last members
of such species may hang on for some decades, but their ultimate
doom is assured.
The findings are the result of a comprehensive
examination of more than a thousand terrestrial species -- plants,
insects, mammals, birds, frogs and reptiles -- in regions representing
about 20% of the Earth's surface. The regions studied are located
in all continents except Asia, and represent a wide variety of
environments: boreal (northern), temperate, and tropical forests,
tundra, grasslands, savannah, deserts. The amount of warming that
was projected in the study was shockingly small. Three projections
were used: 0.8 to 1.7 °C (1.4 to 3.0°F) in the minimal
warming case, 1.8 to 2.0°C (3.2 to 3.6°F) with mid-range
climate change, over 2.0°C (3.6°F) at maximum (Thomas,
2004; Pounds and Puschendorf, 2004).
But with only this rather minimal amount
of warming, and even with an assumed ability to disperse to more
favorable environments, 11, 19, and 33 percent of total species
(in minimal, mid-range, and maximal cases, respectively) will
disappear. Mortality among those species with little or no ability
to disperse will be considerably higher (34, 45, and 58 % in the
respective no dispersal cases). Moreover, the "minimal"
case (0.8 to 1.7 °C/1.4 to 3.0°F) represents the minimum
expected warming by 2050: as the study's authors point out, this
means that this level of extinction is inevitable (Thomas, 2004).
In 50 years, more than 10% of terrestrial species -- at minimum
-- will be on a one-way path to extinction; in 100 years, almost
all those species will be gone.
"Contrary to previous projections,"
the authors note, "[climate warming] (which they attribute
to human activity) is likely to be the greatest threat in many
if not most regions." The study did not examine the "historically
unprecedented" carbon dioxide levels with which organisms
will have to contend, or interactions between climate change and
other ecological threats, which the authors indicate are likely
to be even more severe than climate change in isolation (Thomas,
2004). The message of this study is simple: climate change kills
-- and kills extraordinary numbers of living things -- even when
it is minor.
Global warming would also have had a major impact on the chemistry
and biota of the oceans. Once the ocean began to warm, it would
have begun to stratify. The downwelling of frigid but highly oxygenated
water in polar regions would have slowed, and perhaps stopped.
In today's world, this water is responsible for keeping most of
the ocean (except for its surface layer) oxygenated. Without the
input of large quantities of oxygen, the deep ocean would have
become anoxic, and once anoxic, anaerobic organisms like sulfate-reducers
and methanogens would have replaced the former aerobic inhabitants.
Permian global warming would have had
several additional consequences. It would have increased the rate
of evaporation, making the continents more dry even while making
the atmosphere more humid. It also would have increased the amount
of water that the atmosphere could hold, and, because water itself
is the dominant greenhouse gas, that water would have contributed
to still further global warming. Cloud cover would have increased,
but the role of clouds in determining climate is a matter of continuing
discussion and much serious investigation among scientists, because
clouds on one hand increase Earth's albedo (thus reflecting more
solar radiation back into space) and cool the planet, and, on
the other, increase the amount of warmth trapped beneath them.
It is still unknown whether, on balance, clouds contribute more
to planetary warming or cooling, though the answer is sure to
be different for different types of clouds. As we have already
seen, some clouds -- polar stratospheric clouds -- may have helped
warm the polar regions at the beginning of the Eocene.
More water vapor in the atmosphere, however,
would have meant more precipitation, and this precipitation would
have come in the form of more severe storms. Some continental
areas therefore may have been subject to a kind of double whammy:
drying out more because of increased evaporation, and then periodically
getting hit by powerful storms. This is a prescription for drought
punctuated by flash floods and rapid erosion, and would have imposed
difficult living conditions on the many of the creatures of the
affected areas. Other areas simply received more precipitation,
which ameliorated harsher climates and made arid climates less
so (Retallack, 2003).
In South Africa's Karroo Basin, the additional
precipitation may have been responsible for changing river dynamics
and altering meadering rivers (which sweep in great curves across
their valleys) to braided rivers (which are comprised of many
interwoven strands). Although numerous factors control the specific
shapes of rivers, the amount of flow is an important one, and
braided rivers tend to indicate higher flow rates than meandering
ones. The major extinction of terrestrial plants, whose root systems
tend to anchor soils, is also a likely factor in altering river
shape (Ward, 2000).
Though the study of the effects of warming
on terrestrial species (the Thomas, 2004, study) examines only
present-day global warming, its application to the end-Permian
is obvious. While the initial triggering mechanism is different
-- the anthropogenic production of carbon dioxide in the present
versus direct oceanic warming and indirect CO^2-induced global
warming caused by Traps volcanism at the end of the Permian --
the effects would have been the same. The atmosphere warmed, and
the ocean and the terrestrial surface with it. Huge numbers of
species were not able to survive the changed conditions, and they
died.
The end-Permian methane catastrophe
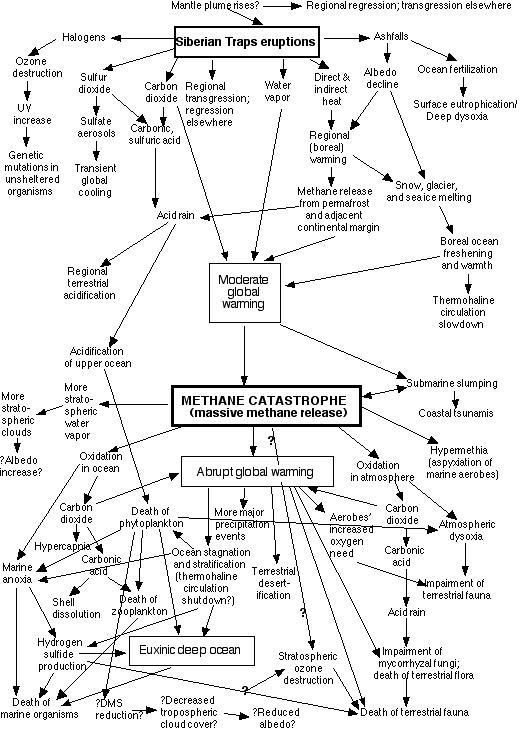
Rising from the Deep:
Continental margin methane may be released in several different
ways.
Trickling or Seeping
Undoubtedly some methane trickles out from dissociating hydrate
and the free gas below it. It presumably exits the sediments via
the escape routes of single chimneys, small cracks and faults.
This escape is presumably relatively steady in some places, more
intermittent in others. This trickling (both of dissolved methane
and small bubbles thereof) is probably the main way oceanic methane
is released today. Although almost all of the continental margin
methane is consumed by methanotrophs living near (within about
5 cm, or 2 inches) the sediment surface, some does exit the seafloor
though "cold seeps."
Cold seeps are places where fresh, cold
water flows from the sediment. The coldness, and the freshness,
of the water, is due to the dissociation of methane hydrate. Hydrates,
remember, are composed of water ice. A given volume of methane
hydrate contains 80% water, and the water is fresh because freezing
excludes salt. Therefore, when methane hydrate dissociates, not
only methane is released. A substantial quantity of fresh water
is released as well. This water can be detected by oceanic probes,
and can be used to locate the cold seeps from which methane also
exits.
A certain amount of methane is also carried
upwards by the warmer fluids produced where ocean floor is being
subducted beneath continents. As ocean plates drop down at active
continental margins, their sediments are heated by the warmth
from the Earth's interior, and compressed by the force of the
two plates -- oceanic and continental -- pushing against each
other. This warmth and compression dewaters clay minerals and
forces their freshwater fluids out and up through the overlying
sediments. These fluids carry high concentrations of both methane
and hydrogen sulfide. As with other methane that is released through
the sediments by trickling, most is consumed by methanogens near
the sediment surface. On the sediment surface, the fluids produce
mud mounds, just as do the methane-bearing fluids from and below
the hydrate layer (Hensen, 2004).
Trickling is the normal mode by which
most continental margin methane is released. Because it furnishes
methane to the sediment close (within about 5 cm, or 2 inches)
to the seafloor at a relatively steady rate, it furnishes a steady
diet for methanotrophs.
Rafting
Some of the methane which trickles out, perhaps due to frigid
seafloor currents, may re-form as hydrate at or near the sediment-water
interface. There it may accumulate as larger chunks, possibly
up to the size of a small house. But as methane hydrate is lighter
than water, there is a limit to how much will accumulate before
it tears free and floats to the ocean surface. As it floats upward,
of course, it will effervesce, and will continue to do so on the
ocean surface until it has completely dissociated. This rafting
process is what provided the Ocean Selector with its complement
of methane hydrate. Rafting, however, is not a significant mode
of methane release.
Venting
Because the free gas below the methane hydrate is at critical
pressure (Hornbach, 2004), changes in the pressure of the overlying
seawater, due to changes in sea level, may cause it to force open
faults in the sediment. Similarly, the warming of the free methane
will increase its pressure, helping pry open the seafloor faults.
Because those faults often cut through the hydrate layer as well
as the overlying sediments, warmer water seeping through the faults
will dissociate hydrate and facilitate the escape of free methane
below. This venting of free methane via faults would continue
as long as the pressure of the free methane and fluids can hold
those faults open.
Faults, it should be noted, are not just
simple breaks in rock or consolidated sediments. They are frequently
highly complex, branched and braided even at the smallest scales.
Thus, when a submarine fault is "pried open," that will
not cause a gaping crack in the seafloor. Instead, just enough
space will be created to allow the escape of gas, and as those
who have ever had a gas leak know, that can be tiny indeed. (Leaky
roofs or ceilings also illustrate the point, but with a liquid
rather than a gas.) With increased pressure, the cracks that comprise
the fault widen; with decreased pressure, they narrow and may
close completely. Thus, depending on the pressure of the free
methane and fluid, more or less of it will be released by this
venting process. Presumably this happens intermittently even in
today's oceans; warming will increase, and eventually substantially
increase, its occurrence.
Venting is a release mode which allows
the release of free methane from below the hydrate and dissociated
methane from the hydrate itself. It produces pock marks and mud
mounds on the seafloor surface. Venting may also be responsible
for the formation of mud volcanoes, though substantial quantities
of dissociated hydrate methane may be required as well. It is
distinguishable from trickling by being more episodic, as the
pressure of free gas forces open faults in the sediment. Trickling
is a slower, more normal mode of release.
Mud Volcanism
Methane hydrates are quite buoyant, and are even, as mentioned
previously, more buoyant than water. But methane hydrates usually
remain stuck in the mud in which they formed. Nonetheless, their
buoyancy, coupled with the buoyancy of the free methane below
them can allow them to push upwards and distort the overlying
strata. Thus one seafloor structure commonly found in association
with methane hydrates is the dome.
Some dome-like structures are the surface
manifestations of subsurface features called diapirs: large, narrow,
roughly cone-shaped columns/pillars that have intruded from below
into overlying sediments. Diapirs form when more buoyant sediments
lie beneath consolidated, but less deformable sediments or sedimentary
rocks. Diapirs are often composed of salt because salt is buoyant
and very easily deformed, especially because it easily absorbs
and frequently contains large quantities of water. But diapirs
of methane hydrate, and even of shale, a sedimentary rock, are
also common.
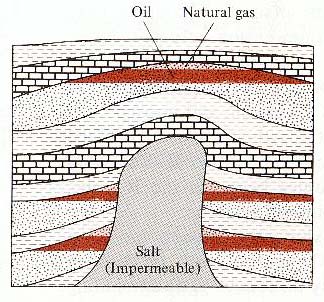 Diapir.
In this case, the diapir is composed of salt. Such diapirs are
common in the Gulf of Mexico and the Persian Gulf. Diapirs push
upwards, distorting the rocks and muds above them and causing
them to "dome." Here the doming provides places where
oil and natural gas can accumulate. ( Ludman and Coch, 1982, Figure
17.38, p. 396)
Diapir.
In this case, the diapir is composed of salt. Such diapirs are
common in the Gulf of Mexico and the Persian Gulf. Diapirs push
upwards, distorting the rocks and muds above them and causing
them to "dome." Here the doming provides places where
oil and natural gas can accumulate. ( Ludman and Coch, 1982, Figure
17.38, p. 396)
On occasion, the pressure of the buoyant
methane hydrate and free methane gas can break through the domal
structure to erupt as a mud volcano. Produced by the dissociation
of large quantities of methane hydrate, mud volcanoes form structures
that resemble typical volcanic cones. But unlike ordinary volcanoes,
they erupt no hot magma or ash. (There are indeed ordinary volcanoes
on the ocean floor, both at the oceanic ridges where new crust
is formed, and in places where what are known as "hot spots"
are present. Some of these ordinary volcanoes are active; many
more are extinct.) Instead, mud volcanoes are the result of water
and gas forcing its way through overlying mud. They form when
sediments containing large amounts of fluid are compressed and
the fluid squeezed out and upwards through faults and cracks in
overlying sediments.
The pressure of the fluid, which is primarily
water, and the highly buoyant methane fractures and shatters more
hardened, consolidated mud, carrying irregular and angular chunks
(mud breccia) upwards, and leaving them imbedded in the softer
muds above, or spewed out on the nearby ocean floor.
Mud volcanoes, when active, can emit
clouds of water, silt, and gas, primarily methane from hydrate.
That the source of the water in this fluid is hydrate is indicated
by its freshness, in contrast to the salinity of the surrounding
seawater. The expelled fluid does mix with the seawater, however,
often producing detectable plumes of water of low salinity and
high methane content in the seawater above.
Using geological dating techniques, scientists
can determine that the mud breccia chunks found on the seafloor
are from older, and formerly deeper, sediments than those in which
they are found, confirming that they were erupted from these undersea
mud volcanoes. The mud chunks smell of the familiar gas hydrogen
sulfide (which provides the odor of rotten eggs), because methane
itself is odorless.
Mud volcanoes, like other volcanoes,
are typically circular when viewed from above, average about a
kilometer (0.6 mile) in diameter, and rise about 100 meters (over
300 feet) above the seafloor. In the Anaximander mud volcano field
in the eastern Mediterranean, the largest volcano is about 2.5
kilometers (1.5 miles) in diameter, and stands about 360 meters
(more than 1000 feet) above the seafloor. Its mud flows cover
an area about 50 square kilometers (about 20 square miles). The
smallest of the mud volcanoes in this field are only about 700
meters (3000 feet) across, and 60 meters (200 feet) high (Woodside,
1998).
Worldwide, there are an estimated 1800
mud volcanoes. In the Arctic, similar conical, volcano-shaped
structures are found in permafrost, both on land and underwater
on the continental margin. They are commonly 30-50 m high, and
as large as 400 m across. Called pingos (or alternatively, boolgoonyakhs!),
they also vent methane-containing fluids, presumably from melted
hydrate.
The eruption of dissociated methane hydrate
from sediment can leave clear evidence of that release. Seismic
images of mud volcanoes reveal that -- unlike ordinary volcanoes
-- strata dip downwards toward the interior of the volcano, toward
the passages where the fluids escaped. This pattern can be explained
by the collapse of overlying strata once hydrate fluids have been
vented, and/or by the weight of some of the erupted sediments
settling back down on the volcano (Woodside, 1998).
Mud volcanism, or simply the venting
of large quantities of methane hydrate, may explain the presence
of crater-shaped structures on the continental slope off Cape
Hatteras, North Carolina. What were thought to be "cracks"
in the slope have turned out to be giant, elongated craters, two
by five kilometers (1.2 by 3 miles) across (Driscoll, 2000). Mud
volcanism is presumably an occasional phenomenon. Once a mud volcano
has erupted and thereby released its underlying store of methane,
further major eruptions are probably unlikely.
Sediment Waves
Just as ripple marks are carved in sand by the action of waves
(both on- and off-shore), so also are sediment waves sometimes
carved in undersea sediments. Sediment waves are caused by the
undersea currents which can move vast quantities of sediment into
ridges and troughs. At Blake Ridge off the Florida/Carolina coast,
the ridges are straight to curved, even crescent-shaped, and may
be as much as 10 kilometers (6 miles) long. These ridges, which
can be as much as 150 meters (yards) in height above their neighboring
troughs, are one to three kilometers (about 0.6 to 2 miles) apart
(Holbrook, 2002).
Methane apparently was rapidly, gradually,
or episodically released from hydrate as the sediment was eroded
and redeposited. The amount of methane released by these sediment
waves (which have little or no hydrate left) can be estimated
by comparison with nearby areas which have not been similarly
disturbed. The amount turns out to have been considerable: some
600 million tons, equivalent to about 12% of the atmosphere's
present content. At Blake Ridge, however, this quantity may have
been released over as much as two and a half million years (Holbrook,
2002), making this release mode non-catastrophic. It seems unlikely
that sediment wave release could produce, or even significantly
contribute to, the major negative carbon isotope excursions seen
in the geological record. Moreover, although the direction and
strength of underwater currents could change dramatically with
changes in sea level or ocean temperature, thereby altering sediment
wave erosional and depositional patterns, neither temperature
nor sea level change directly affects these patterns. Other release
modes are therefore more likely to be responsible for major releases
of methane.
Massive Dissociation
Whereas venting primarily involves the free methane below the
hydrate, massive dissociation involves the hydrate itself. The
term "dissociation" refers to the breakup of the clathrate
structure of the hydrate, those icy lattices that contain the
methane. Once these lattices dissolve, the methane is released.
Small amounts of methane are always being released through dissociation;
massive dissociation refers to significant breakup of the clathrates,
and a major release of methane. This can occur only by the warming
of the sediments, or by their depressurization caused by a fall
in sea level.
On wide and flat continental margins,
where there is no gradient which would allow for slumping, massive
dissociation is likely the only important mechanism for large-scale
methane release. Because the dissociation is the consequence of
pressure or temperature changes, this process is protracted compared
with other modes of methane release, though it can occur rapidly
as measured in geologic time. This rapid release is presumably
greater than the methanotrophs can accommodate, and methane is
released into the water column and then the atmosphere.
Slumping
Slumps (submarine landslides) take place on continental slopes.
There the seafloor gradient is sufficient to allow for such submarine
landslides, under the appropriate conditions. Methane hydrate
can cause or contribute to the instability of continental margins
and the likelihood of submarine landslides in several ways. First,
because the methane hydrate stability zone overlays a zone of
free gas marked by the BSR, the BSR becomes a potentially unstable
boundary layer where slippage can occur.
Second, this boundary layer can be made
even more unstable by the increase of gas pressure produced as
the amount of free methane accumulates below. This results in
the BSR becoming "overpressured." Finally, hydrate dissociation
within the continental slope can weaken the sediment pile, and
the additional water and gas within the sediment provides a low
friction layer on which the overlying sediments can slide. In
addition, some deeply buried seafloor sediments contain silty
sands which can trap astonishingly large quantities of water,
reducing these strata to little more than slurries.
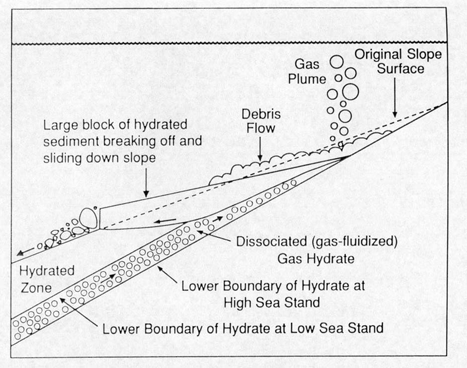 Submarine landsliding as a
result of hydrate dissociation
triggered by depressurization. The depressurization is the result
of falling sea level. This is one suggestion for the cause of
the Second Storegga slide, which occurred where there was significant
glacial rebound after the most recent ice age. Oceanic warming
has been proposed as a separate or perhaps contributory cause.
(Kvenvolden, 1994)
Submarine landsliding as a
result of hydrate dissociation
triggered by depressurization. The depressurization is the result
of falling sea level. This is one suggestion for the cause of
the Second Storegga slide, which occurred where there was significant
glacial rebound after the most recent ice age. Oceanic warming
has been proposed as a separate or perhaps contributory cause.
(Kvenvolden, 1994)
These waterlogged sediments are the consequence
of high rates of sedimentation, and are therefore most likely
to be found in seafloor areas that were near continental ice sheets
during a previous last ice age. With their great weight, continental
ice sheets scrape all soil and sand from the areas they move across,
and grind the rock below to powder (called "rock flour").
Thus, they can transport immense quantities of sand, rock, and
rock flour directly to the ocean, and, upon melting, leave behind
on the land immense quantities of this debris to be later carried
to the ocean by the action of rivers. Ongoing sedimentation can
bury the waterlogged sediments, and result in highly unstable
seafloor conditions extending to depths of hundreds of meters
(yards; Dugan and Flemings, 2000).
Slumping itself may be triggered by an
earthquake, an increase of sediment weight, or the gradual or
sudden dissociation of hydrate in the sediments. The association
between hydrate dissociation and slumping, in fact, was one of
the first dangers of seafloor hydrates to be recognized, now almost
thirty years ago (McIver, 1977). Not surprisingly, slumping is
the most abrupt and potentially catastrophic of the various modes
of methane release.
Continuous and Episodic versus Catastrophic
Release
Certain of the release modes of continental margin methane are
more or less continuous, and seem to represent the usual way that
methane is released from margin sediment. These modes are trickling
and rafting, though rafting is a very minor release mode and only
attracts attention because of its startling unfamiliarity. Sediment
waves presumably develop regularly in various parts of the world
ocean seafloor over long periods of time, and their methane likely
is also released gradually. Venting occurs episodically, as free
methane pressure builds in sediment and then forces its way out
through faults or such other fluid release structures as pockmarks.
Mud volcanism is an occasional, local phenomenon that periodically
injects small quantities of methane into the ocean and atmosphere.
The remaining two modes allow for catastrophic
release. Rapidly warmed or depressurized, great quantities of
methane may be released from hydrate and below in geologically
short periods of time: a few centuries at most. And slumping --
also attributable to warming or depressurization -- can release
huge amounts of methane almost instantaneously. Moreover, the
quantity of methane that can be released in a single event is
vastly greater than can be consumed by methanotrophs.
Submarine landslides
Massive landslides are among the most
rapid and destructive geological processes on the planet. But
since submarine landslides are not visible to the unaided eye
-- and in the murky depths of the ocean can only be seen and photographed
in tiny areas which provide no clue as to their real extent --
a comparison with a highly visible terrestrial landslide may be
useful.
In southern California is the Blackhawk
slide, found on a northern slope of the San Bernardino Mountains.
These mountains, north of the Los Angeles Basin, are part of the
Transverse Ranges and are some of the fastest rising mountains
in the world.
The Blackhawk slide originated on Blackhawk
Mountain, and flowed down Blackhawk Canyon, then emptied on to
the nearby Mojave Desert floor. The slide occurred about 17,000
years ago, but according to the generally accepted scenario for
its formation, it entirely took place in about 80 seconds, a geological
eyeblink. The velocity of the slide is estimated to have at times
exceeded 400 kilometers (250 miles) per hour, with the moving
rock sometimes riding on a highly compressed cushion of air and
at others being fully airborne, more than a hundred meters (yards)
above the ground. The slide left a huge apron of debris 8 kilometers
(5 miles) long, 3.2 kilometers (2 miles) wide, and from 10 to
30 meters (yards) deep (Norris and Webb, 1990).
The Blackhawk Slide, Mojave Desert, southern California.

Yet the Blackhawk slide (which did not
involve the release of methane), despite its awesome appearance,
involved only 1/1000th as much material as a methane release related
slide in the eastern Mediterranean! Called "The Great Slide,"
it is found in the same area as the Anaximander mud volcano field,
a region of abundant seafloor methane release structures. The
Great Slide covers from 1200 to 2200 square kilometers (460 to
845 square miles) and involved at least 550 cubic kilometers (135
cubic kilometers) of sediment (Woodside, 1998). That's enough
to bury Manhattan Island 10 kilometers (6 miles) deep in mud.
Nor is the Anaximander area slide unusual. Huge submarine landslides
are common to the oceans of the world, though fortunately they
do not occur frequently.
Depressurization is the likely cause
of the Storegga ("Great-edge") Slide off the coast of
Norway. Actually, the Storegga Slide is comprised of three separate
slides, one probably about 50,000 to 30,000 years old, the others
dating to about 8000 to 6000 years ago. The cause of the first
slide is unknown, but the cause of the other two likely was related
to the conclusion of the most recent ice age, about 12,000 to
10,000 ago (Bugge, 1988).
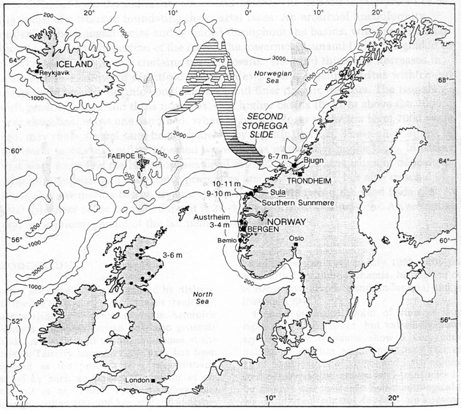 Map of the Second Storegga
Slide. This submarine landslide,
which occurred about 8000 years ago, is one of the greatest underwater
slides known. The black circles mark where deposits from the tsunamis
that were generated by the slide have been found. The nearby numbers
specify minimum tsunami heights in meters (yards). Though not
indicated on this map, tsunami heights reached to over 20 meters
in the Shetland Islands, the unlabeled island group just to the
west of the "A" in Austrheim (Bondevik, 2003). The contour
lines indicate seafloor depth, also in meters. (Map from Bondevik,
1997)
Map of the Second Storegga
Slide. This submarine landslide,
which occurred about 8000 years ago, is one of the greatest underwater
slides known. The black circles mark where deposits from the tsunamis
that were generated by the slide have been found. The nearby numbers
specify minimum tsunami heights in meters (yards). Though not
indicated on this map, tsunami heights reached to over 20 meters
in the Shetland Islands, the unlabeled island group just to the
west of the "A" in Austrheim (Bondevik, 2003). The contour
lines indicate seafloor depth, also in meters. (Map from Bondevik,
1997)
The ice age put a huge weight of ice
on much of the continents of Europe and North America. These great
continental ice sheets were one and a half to three kilometers
(one to two miles) thick. Their colossal weight pressed down on
and warped the crust of the earth in the areas covered by the
ice. At the end of the ice age, the crust gradually returned to
its former position. That process is still going on in various
parts of the world, including Puget Sound area of Washington State.
This process is called glacial rebound.
Glacial rebound occurs because the area formerly under the great
weight of ice is returning to isostatic equilibrium. Isostatic
equilibrium is a big phrase for something which is very familiar.
Consider the rubber ducky floating in the bathtub. The duck is
in isostatic equilibrium with the water.
But push that duck under water: it pushes
against your hand, wanting to float free. The duck is no longer
in isostatic equilibrium with the water. It takes the continuous
application of the force of your hand to keep it under the surface.
When you release the duck, it pops up into the air above the water
surface, then splashes back down, as it returns to isostatic equilibrium.
The crust of the earth is also generally
in isostatic equilibrium with the mantle rock lower down. That
may seem surprising, because we don't usually think of the ground
we walk on as floating. For unlike the water in the tub, the earth
seems solid. And, generally speaking, it is. But while the crust
doesn't exactly float, it does have a bit of give. So when a great
weight is put on the crust, it sags, ever so slightly if the weight
is small, but more if the weight is considerable.
Dams, for example, hold back a great
deal of water, and the weight of that water can actually cause
the underlying crust to buckle. The amount of buckling is small,
but it can cause minor -- or quite major -- consequences. Minor
effects include tiny, virtually undetectable earthquakes. Larger
earthquakes are also possible, however, depending on the nature
of the crust upon which the dam and its reservoir sit. In India,
such an earthquake was the probable trigger for the 1993 Killari
earthquake, centered near a dam on the Lower Tirna River, which
leveled 52 villages and killed almost 10,000 people in an area
which had previously had been considered a seismically stable
part of the subcontinent.
In the case of the Storegga slides, the
weight upon the crust was ice. Earthquakes, perhaps only small
ones, presumably accompanied both the pressing down of the crust
as the great European ice sheet accumulated, and when the pressure
was released during deglaciation, The ice sheet covered not only
that land currently above sea level, but also, because the ice
accumulation had significantly lowered sea level, areas of the
continental margins now well under water.
At the end of the ice age, between 12,000
and 10,000 years ago, the great continental ice sheets melted.
Then, probably accompanied by periodic earthquakes, the continental
crust began to rebound. At least twice, perhaps triggered by such
a quake, a huge portion of the methane hydrate-laden Storegga
area sediment off the Norwegian coast gave way.
It is possible that the first of the
slides also was caused by an episode of warming. We do know that
even during the period of greatest glaciation, temperatures, though
cold, could be quite variable. But our knowledge of both the timing
of temperature changes during the ice age, and the timing of the
first Storegga slide are too limited to make any specific connections
between the two. Nonetheless, oceanic warming may been the cause
of the other suggested trigger for the Storegga slides: the dissociation
of continental margin methane hydrate. (That a huge quantity of
methane may have been released is revealed even today in the landslide
debris, which contains about a hundred craters, some larger than
three kilometers (two miles) in diameter. It is possible, however,
that some of these structures simply record the escape of water
trapped below the landslide material as it settled.)
The total amount (in all three Storegga
slides) of sediment that slid is estimated at about 5500 cubic
kilometers (1340 cubic miles), enough to bury Manhattan Island
to a depth of almost 95 kilometers (almost 60 miles!) or San Francisco
or Boston to a depth of 45 kilometers (26 1/2 miles) deep. Some
of this sediment slid as far as 800 kilometers (500 miles) down
and across the adjacent ocean floor, presumably by hydroplaning
on an incompressible slick of water (Elverkøi, 2004). The
slides produced tsunamis whose debris is now found in coastal
Norwegian lakes 18 meters (yards) above sea level.
An estimated 350 billion metric tons
of methane was released in these slide events, both from dissociated
methane hydrate and the free gas that lay below them. This amount
of methane contains some 263 billion metric tons of carbon, equivalent
to about 1/3 the total carbon in the atmosphere, making its release
roughly equivalent to the amount of anthropogenic carbon released
into the atmosphere since the beginning of the industrial age.
(Had these events been part of a "slump cascade," with
several similar slides in close succession, the consequences are
likely to have been much more severe than the minor transient
warming that probably ensued.)
What is known as the second Storegga
Slide (about 7300 years ago) produced a tsunami along the coasts
of both Norway and Scotland, pointing to an additional consequence
of methane-related submarine landslides. (Tsunamis generally come
in several waves, minutes apart, with the higher waves coming
first. Here I use the single form, tsunami, though the plural
form would also have been correct.) Geologists have found evidence
for this tsunami in lake and pond tsunami deposits on nearby continental
coasts. (These deposits are easily identified because they contain
sand and chunks of rock swept and torn from the ocean bottom,
rather than the mud and silt sediments that normally accumulate
on the bottoms of small lakes and ponds.)
Runup heights reached 10 to 11 meters
(yards) above what would then have been high tide level along
parts of the Norwegian coast and remained high (3 to 4 meters)
even in Norway's far north (Bondevik, 1997). The tsunami even
reached the eastern coast of Scotland, more than 900 kilometers
(540 miles) from the Storegga slide itself. Traveling at a speed
of about 360 kilometers per hour (215 mph), the tsunami ran up
4 meters (12 feet) above high tide level (Dawson, 1988). (It should
be noted that these are minimum tsunami heights. The top meter
or so of a tsunami seems to carry little debris, so leaves no
evidence behind. In addition, if measured down to low tide level,
tsunami heights would have been 2 to 3 meters greater because
those heights are usually measured from where the tsunami left
its deposits down to the high tide level.)
At the Shetland Islands, just over 100
kilometers (60 miles) from the northeast tip of Scotland
and about 300 km (180 miles) from the closest part of the Storegga
slide tsunami run-up height reached 20 to 25 meters (yards).
The tsunami evidence is seen in lake deposits and peat outcrops,
where a sand layer, ripped-up chunks of ancient peat, pieces of
branches and tree trunks, and bowling ball sized rocks. Carbon
dating of peat debris and sticks in and above the tsunami deposit
has confirmed that the second Storegga slide took place about
7300 years before the present (Bondevik, 2003).
Tsunamis are not extinction mechanisms,
though occasionally they may wipe out a species of coastal organisms
that has a quite limited range. Coastal inhabitants in the affected
areas, however, may suffer greatly.
The Length of the Permian-Triassic
Carbon Isotope Excursion
Supportive evidence for the rapid methane
release scenario comes from independent estimates of the length
of the extinction event itself. Using the various cycles of the
obliquity (ovalness) of the Earth's orbit and the direction and
tilt of its axis (cycles referred to as the Milankovitch cycles),
the main Permian-Triassic faunal changes seem to have occurred
within a period of less than 60,000 years, and perhaps as few
as 8000 (Rampino, 2000). This estimate compares favorably with
the estimate for the length of the negative carbon isotope excursion
of less than 30,000 years (Rampino, 2000).
The fungal spike at the end-Permian/Early
Triassic, in the Israeli rocks, is estimated to have lasted only
as long as 54,000 years and perhaps less, based on the estimated
rate of sedimentation for such shallow-marine sedimentary rocks
(Eshet, 1995). A calculation of the length of the fungal spike
in the southern Alps yields 25,000 years or less (Eshet, 1995,
using the data from Sweet, 1992). In China, the Meishan section
yields an estimated duration of about 200,000 years for the fungal
abundance (Ouyang and Utting, 1990, cited in Eshet, 1995). The
"acritarch explosion," appears to have occurred in a
period of about 200,000 years, though they were declining in numbers
after 100,000 years (Eshet, 1995, Figure 2). Finally, as determined
by carbon isotopes in kerogen (fossilized organic matter), the
negative isotopic excursion (here measured at about 3.6
± 1) lasted from 50,000 to 100,000 years before primary
productivity was restored (Wang, 1994).
All of these estimates match up well
with the roughly 10,000 to 20,000 years for a carbon isotope excursion
produced by the dissociation of hydrate methane as seen for the
Late Paleocene Thermal Maximum, and for the roughly 100,000 to
200,000 years for recovery to normal values. It is important to
emphasize that these time estimates derive from quite unrelated
lines of evidence: the cycles of Earth's orbit and tilt, carbon
isotopes from kerogen, how long fungi spiked and waned, how long
unusual numbers of acritarchs were around. Though other suggested
scenarios for the cause of the end-Permian extinction may be found
to be compatible with these time estimates, it is only the margin
methane proposal which independently generated such timelines
(based on the LPTM and computer modeling), and enjoys several
independent lines of support by way of confirmation.
The continental margin methane release
scenario also meets other previously suggested standards for evaluating
the various end-Permian extinction theories. In 1998, Bowring
and his co-authors proposed criteria for evaluating end-Permian
extinction theories. They specified that "The following seven
observations must be accommodated in any model for the Permian-Triassic
extinction" (the ···bulleted
items after each observation indicates how the methane catastrophe
scenario meets that particular criterion):
(i) "the widespread evidence of
anoxia in latest Permian-earliest Triassic deep-ocean sediments,
including accumulation of massive amounts of organic carbon."
···The massive release of seafloor
methane does cause anoxia in the water column, and the accumulation
of large amounts of carbon in the sediments would be an immediate
effect of the extinction. The extended anoxia and black shale
accumulation noted by Isozaki (1994; 1997) was probably due to
stagnant ocean conditions fostering an anaerobic deep marine biota.
Anaerobic decomposition takes place much more slowly, and less
efficiently, than decomposition by aerobes. More organic debris
from surface waters would therefore have reached the seafloor.
(ii) "the ^13C isotopic excursion
at or near the Permian-Triassic boundary in both marine and terrestrial
ecosystems, although the precise relation between peak extinction
and the isotopic shift remains unclear."
···Biogenic seafloor methane explains
the carbon isotope excursion because it is so depleted in ^13C.
(iii) "evidence for anoxia in nearshore
settings at or close to the Permian-Triassic boundary, coincident
with a major earliest Triassic transgression."
···Nearshore anoxia can be explained
by the same methane release mechanisms as previously described.
In addition, the activity of anaerobic methanotrophs in the top
few centimeters (two inches) of sediment produces hydrogen sulfide,
which itself is toxic and draws down dissolved marine oxygen.
The Early Triassic transgression mentioned by the authors may
reflect the sinking of northern Laurasia after the eruption of
Siberian Traps magma, perhaps with a small contribution from the
melting of mountain glaciers. However, as noted previously, transgression-regression
data may not be reliable, and in this case the data is from twenty
years ago (Bowring, 1998, relies on Holser and Magaritz, 1987,
whose data comes from earlier sources).
(iv) "patterns of extinction consistent
with hypocapnia or CO¸2 poisoning."
···Presumably the authors refer to
hypercapnia, carbon dioxide excess, rather than hypocapnia, having
too little carbon dioxide. Methane, as noted numerous times, is
quickly oxidized to carbon dioxide.
(v) "the age of the Siberian Traps
and extinction being the same within error."
···With the Traps as trigger, permafrost
and Arctic continental margin methane would have started being
released immediately.
(vi) "a lack of evidence for a latest
Permian glaciation."
···The methane release proposal posits
a possible short-term cooling, not from the methane itself, but
from the Siberian Traps volcanism. This cooling episode would
have been over within a matter of years (not sufficient for glaciation),
and the long-term warming would have quickly become dominant.
Further pulses of Traps volcanism could have caused additional
short-term cooling, but these would have been minor blips in the
overall warming pattern. The methane release theory does not postulate
any glaciation for the Late Permian or Early Triassic.
(vii) "and sudden climate warming
at and after the boundary."
···This warming would have been caused
by the greenhouse gas methane, its successor gas carbon dioxide,
and the increased presence in the atmosphere of water vapor, the
result of the warming.
Thus the continental margin methane release
proposal meets all of the criteria suggested by Bowring and his
co-workers (1998).
The continental margin methane release
theory relies on Siberian Traps volcanism as the trigger for methane
release. But if both Siberian Traps volcanism and margin methane
release were involved in the end-Permian extinction, why should
the Siberian Traps be considered as having the subsidiary rather
than the major role in the extinction? What if the methane release
was basically just another minor consequence of the Traps volcanism,
and Traps volcanism was in fact mainly responsible for the end-Permian
extinction?
1. As far as we know, volcanism is limited
in its effects. Other substantial eruptions have -- at most --
only produced limited local or regional effects. Subaerial eruptions
(surface eruptions, that is, those which occur "under the
air" rather than underwater) can produce cooling on a regional
and perhaps hemispheric scale, but that cooling is short-term:
a year or so. Submarine eruptions may have the power to warm nearby
waters, but the effectiveness of that heating would be limited
if the eruption occurred in the midst of large ocean.
In smaller oceans which are partly closed
off from circulation with larger oceans, the effect of submarine
eruptions appears to be magnified, as with the North Atlantic
Igneous Province and/or Caribbean eruptions which occurred at
the Latest Paleocene Thermal Maximum, and may have triggered the
hydrate methane release of that time. On the other hand, the far
greater submarine eruptions that created the underwater Ontong-Java
Plateau in the southwestern Pacific around 100 million years ago
apparently had no such effect. Occurring in a much larger and
more open body of water, there was no catastrophic release of
methane, and no effect on living things was noted in the fossil
record.
2. Even the Deccan Traps eruptions of
66 million years ago seem to have had little effect on the biota
of the time. This eruptive sequence may be the one most comparable
in size and location (near the continental margin) to the Siberian
Traps eruptions, but its biological consequences, if any, were
minimal. It came too early for the end-Cretaceous extinctions,
for which the Alvarez impact scenario seems quite an adequate
explanation.
The Deccan Traps eruptions, in addition,
do not seem to have triggered any release of continental margin
methane. This may be due to their location at the time of their
eruption. India was at the time an island continent, moving across
the Indian Ocean after having broken away from Gondwana. It was
approaching the Asian continent, with which it would collide in
about another fifteen million years. It was in the open ocean,
at about equatorial latitude. This location would not have caused
any serious disruption of global thermohaline circulation. The
Siberian Traps, by contrast, erupted in a much more oceanographically
and climatologically vulnerable part of the world, where they
increased solar heat absorption by reducing reflectivity (albedo),
melted sea ice, warmed seawater, and disrupted global thermohaline
circulation.
Further, the Deccan Traps erupted at
the end of a period of protracted global warmth. The quantity
of seafloor methane hydrate would have been much less than that
at the end of the Permian, which followed the longest ice age
of the Phanerozoic. Thus even as a trigger for another, greater
environmental disaster -- the release of hydrate methane -- the
Deccan Traps had poor timing and bad "location, location,
location."
3. By contrast, large-scale methane release
can indeed produce serious global consequences. It has great potential
as a greenhouse gas for warming the planet, and, of course, it
is quickly oxidized to carbon dioxide. Thus, for warming the planet,
large quantities of methane are an excellent, readily-available
resource. In addition, that methane has the ability to directly
cause oceanic anoxia, and to reduce the level of atmospheric oxygen
by interfering with the activity of marine and terrestrial photosynthesizers.
Therefore, methane has the potential to produce more significant,
global, and protracted consequences for Earth's organisms than
did Siberian Traps volcanism alone.
Nonetheless, the direct ocean warming
effects of Siberian Traps volcanism, together with the indirect
warming from the release of carbon dioxide and decreased albedo,
account for some of the characteristics that make the end-Permian
methane release different. Massive release of hydrate methane
seems only to have occurred at rare intervals during the past
hundreds of millions of years. The period of the Late Paleocene
Thermal Maximum, and the oceanic anoxic events early in the Mesozoic,
as well as possible releases prior to the final episode at the
end of the Permian, may represent the few occasions when such
massive releases took place. But it is the location and volume
of Siberian Traps volcanism which makes the end-Permian release
unique, and likely the greatest methane catastrophe of the Phanerozoic.
Sufficiency
"whom shall we find sufficient?" (Milton, Paradise Lost,
Book II, lines 403-04)
In the case of the various theories about the end-Permian extinction,
what shall we find sufficient?
Recently there have been a number of
new and revived proposals regarding the cause(s) of the end-Permian
extinction. These include methane explosions resulting from the
expulsion of dissolved methane from ocean waters (Ryskin, 2003),
a "champagne cork-popping" of the planet that produces
continental flood basalt events (Morgan, 2004), and another resuscitation
of the discredited impact scenario (Basu, 2003).
The first of these theories starts with
an allegedly anoxic ocean. The anoxia allows methane to reach,
or almost reach, saturation levels (where the seawater has as
much dissolved methane as it can hold) in the ocean. Then, perhaps
triggered by an earthquake, volcano, warm current, or other disturbance
-- or without any external cause at all -- this gas suddenly erupts
from the ocean, much like soda from a shaken pop bottle. Ignited
by lightning, the methane devastates life on land, and the Earth
is plunged either into a period of cooling (caused by sun-obscuring
firestorms) or warming (caused by the increased carbon dioxide
in the atmosphere)(Ryskin, 2003).
The proposal cites as an example of such
explosive effervescence the eruption of volcanic Lake Nyos in
Cameroon, Africa, in 1986, where carbon dioxide that had accumulated
in the bottom of the lake blasted out in a gas-water fountain
for several hours. The ground-hugging carbon dioxide then flowed
downhill, asphyxiating people and cattle. (There was no combustion
explosion, of course, because carbon dioxide, being fully oxidized,
cannot burn. The explosive power of the Nyos eruption came from
the decompression of gas-charged water, just as when a shaken
can of soda pop is decompressed by opening it.)
Despite meticulous attention to the quantitative
and physical aspects of such an eruption, the proposal neglects
an essential biological dimension. An anoxic ocean is not necessarily
a biologically empty ocean. In fact, it seems highly unlikely
that, as methane quantities increased in the ocean, they would
not be consumed by bacterial and archaeal methanotrophs. Even
if such organisms were rare or absent prior to the development
of anoxia, they would quickly expand into such an environment
as dysoxia increased. Since the production of methane in the seafloor
is relatively slow, the expansion of methanotrophy would be able
to keep pace. The concentrations of methane postulated by the
theory would never develop, and therefore the methane explosions
it proposes could not occur.
The Lake Nyos analogy is inappropriate
because the gas in that volcanic lake is primarily carbon dioxide.
While carbon dioxide may be consumed by photosynthetic organisms,
these organisms only exist near the water surface where they can
obtain light, and could not have lessened the amount of carbon
dioxide that had seeped into the deep waters of the lake from
the magma source below. Thus critical quantities of carbon dioxide
can accumulate in volcanic lakes, but critical quantities of methane
are unlikely to accumulate in the ocean.
There's an additional problem with this
end-Permian worldwide conflagration proposal: where's the soot?
At the end of the Cretaceous, there indeed was a worldwide conflagration,
ignited by the extreme heat from the impact. Though the fires
may have spared some areas (Belcher, 2003), it does seem that
they were global in reach, and they did leave evidence of their
destruction: soot found in numerous localities around the planet,
exactly at the K-T boundary (Paine, 1999; Kring and Durda, 2003).
Methane, of course, is always advertized as a "clean-burning"
fuel and so would not have left its own traces, but forested areas
along coasts could not have been immune from firestorms in nearby
oceans -- firestorms which, according to the theory's proponent,
may have occurred repeatedly. But there's no soot in the Permian-Triassic
boundary sections. And no soot, no worldwide conflagration(s).
The second theory proposes that the accumulation
of gas associated with magma rising through Earth's mantle blasted
out a chunk of the planet's crust at the start of a continental
flood basalt (large igneous province) eruption. As with the oceanic
methane explosion theory, this theory relies on gas coming out
of solution. In this case, the gas is carbon dioxide, coming out
of melted rock (magma) as it approached to within about 80 kilometers
(50 miles) of the surface. The theory proposes that this gas would
blast a large column of overlying rock into the atmosphere, spewing
gas and rock fragments over the planet's surface, laying waste
to some terrestrial organisms even before the inevitable greenhouse
conditions set in. The pressure of the rock around the blast hole
would quickly slam it shut (Morgan, 2004).
The authors of this proposal label this
mode of eruption a "Verneshot" after the method of escape
from the interior of the planet by the adventurers in Jules Verne's
"A Journey to the Center of the Earth." In this famous
late nineteenth French science fiction novel, the adventurers
are rafted upwards on the magma of an erupting volcano.
Unfortunately, the Verneshot theorists
attribute the end-Cretaceous extinction of the dinosaurs to a
Verneshot associated with the Deccan Traps eruption (Morgan, 2004).
But as the Cretaceous-Tertiary extinction came after that eruption,
and the attribution of the extinction to an extraterrestrial impact
(the Alvarez scenario) has been generally accepted, this claim
undercuts the credibility of the theory. In addition, the theory
fails to provide any evidence of either the former holes from
which the purported rock columns were ejected or the impact sites
where the rock column projectiles landed.
The third proposal attempts to breathe
life into the end-Permian impact scenario. New evidence, in the
form of meteorite fragments from Antarctica, forms the basis for
the reassertion of an end-Permian impactor. While the meteorite
from which the fragments derive is a standard variety (a chondrite,
the same as for the end-Cretaceous impact), the fragments contain
unusual metal grains. Some of these tiny grains are composed of
iron, nickel and silicon (Fe-Ni-Si), others are almost pure iron
(Basu, 2003).
The proposal again invokes the purported
discovery of Permian-Triassic boundary fullerenes (Becker and
Poreda are co-authors of this paper as well as that of Becker,
2001; see above, Impact Theory) for support, though as pointed
out in a accompanying commentary (Kerr, 2003), none but Becker
has been able to find such fullerenes. The discovery of alleged
(and much disputed) shocked quartz grains is also employed as
confirmatory evidence. The use of such disputed evidence apparently
has failed to convince most scientists who were asked their opinions,
and the ability of almost pure grains of iron to survive for a
quarter of a billion years without weathering has provoked the
response, "'It's astonishing, it's incredible, it's unbelievable'"
(Kerr, 2003; the quotation is from meteor specialist Jeffrey Grossman
of the United States Geological Survey).
(Becker and Poreda have continued to
provide evidence for an end-Permian impact, and that evidence
continues to be questioned. In 2004, Becker and Poreda and colleagues
asserted that seismic imaging and gravity measurements from the
northwestern continental margin of Australia displayed evidence
of a buried end-Permian impact crater. Drill cores from the alleged
impact structure, labeled the Bedout ("bedoo," as in
whoop-bedoo!) Crater, have purportedly revealed nearly pure silica,
fractured and shocked feldspar, and glass spherules in broken,
angular rocks called breccias. The scientists indicate that these
features imply the presence of a large melted area (a melt sheet)
produced by the alleged impact.
In addition, the seismic and gravity
data, they suggest, also reveal the presence of a common impact
crater attribute, a central uplift or peak [Becker, 2004]. Central
peaks are frequently found in impact craters; even the modest
magnification provided by binoculars or small telescopes reveals
many central peaks in lunar craters. Becker and Poreda and colleagues
argue that this alleged central uplift helps define a crater "similar
in size to Chicxulub," the crater that marks the end-Cretaceous
impact. They further assert that although the purported impact
crater is not antipodal -- directly opposite -- to the site of
Siberian Traps volcanism, the impact may have triggered that event.
All told, "The location, size, and age of the Bedout crater
can account for reported occurrences of impact debris in Permian-Triassic
boundary sediments worldwide."
The only direct evidence offered for
the age of the Bedout structure, however, is a single feldspar
grain, though its age is similar to that from another grain extracted
years earlier. The age dating for both grains has a high degree
of uncertainty: some four and a half million years plus or minus
for the new grain, some five million for the older grain. Moreover,
the ages of the two grains differ by almost three million years
[Becker, 2004]. In other words, even assuming that the age-dating
is accurate, the uncertainty in the timing of the purported impact
is about 12.5 million years.
Geologists who study impacts are unconvinced
by the evidence presented. Both Bevan French and Christian Koeberl,
both impact petrologists (those who study impact rocks), doubt
the adequacy of the offered proof. According to French, "'There's
no convincing evidence for an impact origin,'" while Koeberl
stated, "'I see nothing that would convince me there was
an impact'" [Kerr, 2004]. A third impact petrologist, Richard
Grieve, wondered where the indications of flow were in the allegedly
once-molten impact rock, and questioned whether Becker's identification
of a purported shock-produced mineral is actually maskelynite
[a feldspar glass], as claimed.
The assertion by Becker that the purported
Bedout impact "can account for reported occurrences of impact
debris in Permian-Triassic boundary sediments worldwide"
raises additional questions. The "reported occurrences"
have all been challenged; the "worldwide" distribution
of impact evidence, therefore, is a worldwide distribution of
dubious evidence. A widespread scattering of sparse, poor quality,
questionable evidence hardly makes for a convincing case, however
enthusiastic its proponents may be.
The invocation of the end-Cretaceous
impact as a possible cause of the Deccan Traps eruptions in support
of a similar Bedout impact-Siberian Traps connection [Becker,
2004], while not central to the Becker argument, also raises questions
about the way evidence has been pieced together. As note previously,
there is no connection between the end-Cretaceous impact and the
Deccan Traps: the Deccan Traps preceded the impact by 300,000
to as much as 500,000 years. In conjecturing that there may be
two similar impact/massive volcanism geologic sequences, Becker
succeeds only in undercutting her own case that there may have
even been one, that of Bedout and the Siberian Traps. But that
case seems extremely unlikely.
A 2004 paper in Geology asserts that
there is indeed no evidence for an end-Permian impact, either
from helium isotopes or from platinum group (the "platinum
group" includes the elements ruthenium, rhodium, palladium,
osmium, iridium, and platinum, which have similar chemical behavior)
isotopes which would have provided a clear indication of debris
from an extraterrestrial impactor. The authors studied Permian-Triassic
boundary rocks found in Austria and Italy [Koeberl, 2004].)
The evidence we now have regarding end-Permian
events, and their connection to the end-Permian extinction seem
quite adequate to explain that extinction. Traps volcanism initiated
the process, helping shut down thermohaline circulation and warming
the globe, including releasing continental margin methane. The
methane release completed the transition to anoxia in the deeper
ocean, and contributed to dysoxic conditions in coastal areas.
The increased acidity of rainfall, ocean-eutrophying volcanic
ashfalls, toxic gas in the ocean, and occasional bursts of hydrogen
sulfide from the shallow seafloor made additional contributions
to the increasingly unpleasant and inhospitable ecological conditions.
A serious depletion of atmospheric oxygen may also have made its
deadly contribution.
Most important, however, may have been
the rise of global temperatures, initiated by carbon dioxide from
Traps volcanism, and followed by the high atmospheric levels of
methane and its successor greenhouse gas carbon dioxide. The new
projections of the near-future impact of current global warming
indicate that even the heating of the planet by a few degrees
can have extremely lethal consequences (Thomas, 2004). What other
killing mechanisms are necessary? None.
CONTINUE TO NEXT SECTION (Early
Triassic Aftermath)
RETURN
TO CONTENTS





